Frontiers |
您所在的位置:网站首页 › dengue fever乐队 › Frontiers |
Frontiers
REVIEW article
Front. Immunol., 06 July 2022Sec. Viral Immunology
Volume 13 - 2022 |
https://doi.org/10.3389/fimmu.2022.889196
Dengue Infection - Recent Advances in Disease Pathogenesis in the Era of COVID-19 Yean Kong Yong1*† Yean Kong Yong1*†  Won Fen Wong2† Won Fen Wong2†  Ramachandran Vignesh3† Ramachandran Vignesh3†  Indranil Chattopadhyay4† Indranil Chattopadhyay4†  Vijayakumar Velu5,6† Vijayakumar Velu5,6†  Hong Yien Tan7† Hong Yien Tan7†  Ying Zhang8† Ying Zhang8†  Marie Larsson9† Marie Larsson9†  Esaki M. Shankar10*†1Laboratory Centre, Xiamen University Malaysia, Sepang, Malaysia2Department of Medical Microbiology, Faculty Medicine, University of Malaya, Kuala Lumpur, Malaysia3Preclinical Department, Royal College of Medicine Perak (UniKL RCMP), Universiti Kuala Lumpur, Ipoh, Malaysia4Cancer and Microbiome Biology, Department of Life Sciences, Central University of Tamil Nadu, Thiruvarur, India5Division of Microbiology and Immunology, Emory Vaccine Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States6Department of Pathology and Laboratory Medicine, Emory National Primate Research Center, Emory University, Atlanta GA, United States7School of Traditional Chinese Medicine, Xiamen University Malaysia, Sepang, Malaysia8Chemical Engineering, Xiamen University Malaysia, Sepang, Malaysia9Molecular Medicine and Virology, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden10Infection Biology, Department of Life Sciences, Central University of Tamil Nadu, Thiruvarur, India Esaki M. Shankar10*†1Laboratory Centre, Xiamen University Malaysia, Sepang, Malaysia2Department of Medical Microbiology, Faculty Medicine, University of Malaya, Kuala Lumpur, Malaysia3Preclinical Department, Royal College of Medicine Perak (UniKL RCMP), Universiti Kuala Lumpur, Ipoh, Malaysia4Cancer and Microbiome Biology, Department of Life Sciences, Central University of Tamil Nadu, Thiruvarur, India5Division of Microbiology and Immunology, Emory Vaccine Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States6Department of Pathology and Laboratory Medicine, Emory National Primate Research Center, Emory University, Atlanta GA, United States7School of Traditional Chinese Medicine, Xiamen University Malaysia, Sepang, Malaysia8Chemical Engineering, Xiamen University Malaysia, Sepang, Malaysia9Molecular Medicine and Virology, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden10Infection Biology, Department of Life Sciences, Central University of Tamil Nadu, Thiruvarur, IndiaThe dynamics of host-virus interactions, and impairment of the host’s immune surveillance by dengue virus (DENV) serotypes largely remain ambiguous. Several experimental and preclinical studies have demonstrated how the virus brings about severe disease by activating immune cells and other key elements of the inflammatory cascade. Plasmablasts are activated during primary and secondary infections, and play a determinative role in severe dengue. The cross-reactivity of DENV immune responses with other flaviviruses can have implications both for cross-protection and severity of disease. The consequences of a cross-reactivity between DENV and anti-SARS-CoV-2 responses are highly relevant in endemic areas. Here, we review the latest progress in the understanding of dengue immunopathogenesis and provide suggestions to the development of target strategies against dengue. IntroductionDengue is an infectious disease transmitted between humans by Aedes mosquitoes, especially across the tropical and subtropical latitudes afflicting ~400 million people annually, of which 100 million manifests clinically (1). Estimates by the World Health Organization (WHO) suggest that the global consequence of dengue is exponentially increasing and almost half of the global population is at risk for contracting the infection (2). Dengue is caused by at least four different dengue virus (DENV) serotypes, DENV1, DENV2, DENV3, and DENV4. In recent years, most endemic countries, e.g., Asia-Pacific and Latin American nations, are reporting almost all the four different DENV serotypes (3), which altogether cause ~20000 deaths annually (4). The surge in endemicity is attributed to rapid urbanization, increasing population density and a rise in vector-breeding sites (5). Aedes aegypti (A. aegypti) represents the major vector that transmits dengue in urban areas, whereas the density of A. albopictus, the secondary vector, is dramatically expanding globally (6, 7). Given the context of global warming, the environment appears to be appropriate for the breeding of Aedes mosquitos, that in turn, would drive the dissemination of the dengue disease further (8). DENV is a member of the Flavivirus genus of the Flaviviridae family. DENV has a spherical shape with icosahedral symmetry. It is a single-stranded positive sense RNA virus with a genome size of ~11 kb (9). It has a single long open reading frame (ORF) that encodes for three structural and seven non-structural (NS) proteins. The structural proteins are capsid (C), pre-membrane/membrane (prM/M), and envelope glycoproteins (E), and the NS proteins are NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. The NS proteins are not present in the virion, but contribute to viral replication and immune evasion within an infected cell (10–12). Of all the NS proteins, only NS1 is displayed on the infected cell surfaces, and is eventually secreted into the systemic circulation making it an appropriate diagnostic marker. While detection of DENV genomic RNA (by RT-PCR) and NS1 are the mainstay of laboratory diagnosis, detection of NS1 has an edge over the detection of DENV genomic RNA. Albeit being highly sensitive, the RT-PCR viral detection rate has a finer window period where detection rate appears to drop dramatically by day 4 onwards following the onset of clinical symptoms (13, 14). Conversely, NS1 can be detected in the serum for a wider time range, viz., from the first day of symptom onset, with the concentration average of 2 µg/ml (can reach as high as 50 µg/ml in the same cases) (15) that remains detectable between 9 and 18 days (16, 17). Furthermore, the level of NS1 appears to correlate with disease severity, rendering it an ideal biomarker, both for diagnosis as well as prognosis in dengue (12, 18, 19). Dengue infection results in clinical manifestations ranging from a predominantly asymptomatic or a symptomatic, mild undifferentiated febrile illness to severe life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) that can be fatal (6). The hallmarks of severe dengue are coagulopathy and leaky vasculature that eventually can lead to life-threatening hemodynamic shock and organ failure (20, 21). Evidence suggests that young age, female biological sex, high body-mass index, virus strain, and genetic variants of the human MHC class I–related sequence B, and phospholipase C epsilon 1 genes could serve as risk factors for development of severe dengue (6). The human and economic burden caused by dengue fever remains enormous as specific antiviral drugs, or effective vector-control mechanisms is lacking. Although no specific treatment is available, prompt hospital admission, triage, and fluid restoration are critical to prevent death (22). In 2016, (CYD-TDV) DengVaxia®, a tetravalent vaccine was licensed to prevent severe secondary dengue in seropositive individuals. However, the vaccine was not recommended for seronegative individuals as the levels of vaccine-induced antibodies reportedly decreased over time (23). The current review will focus on some of the hitherto poorly understood disease immunopathogenesis mechanisms as well as potential interventions against DENV infection. Dengue Immunopathogenesis: A Brief OverviewThe onset of severe dengue often occurs during the defervescence stage after peak viremia suggesting that the host immune responses are implicated in viral clearance (6, 24), inferring that life-threatening dengue involves a complex interplay between virus and the host (25). Natural infection with one of the serotypes confers long-lasting immunity to subsequent infection with the same serotype. However, subsequent infection with heterotypic serotypes often results in severe immunopathological manifestations, triggered early during the course of disease (26). This, at least in part, could be attributed to a phenomenon known as original antigenic sin that engenders ineffective T and B cell responses and potentially harmful manifestations, particularly during secondary infection. The complex interplay between these factors may eventually lead to both antibody-dependent enhancement (ADE), antibody-dependent cellular cytotoxicity (ADCC), cytokine storm (hypercytokinemia), aberrant activation of the complement system (CS), as well as endothelial dysfunction, culminating in severe clinical dengue (27, 28). Original Antigenic Sin and Antibody-Dependent EnhancementAlthough both T and B cell responses play a paramount role in combating DENV infection (29), they could be pathological during secondary infection due to original antigenic sin. Because the four DENV serotypes share ~80% homology in amino acid sequences, cross-reactivity is common (30). Hence, during a heterotypic infection, the preexisting memory T and B cells rapidly become activated to proliferate to enter into the effector phase (26). As protective adaptive immunity is more efficient against homotypic than heterotypic reinfection (31), seeing that cross-reactive responses may have suboptimal avidity and affinity towards the epitopes of the secondary-infecting virus (27). These cross-reactive T cells often exhibit lower cytotoxicity yet secreting higher abundance of several pro-inflammatory cytokines (32), rendering viral control ineffective as well as exaggerated release of pro-inflammatory cytokines leading to cytokine storm and endothelial dysfunction (26, 33) (Figure 1). FIGURE 1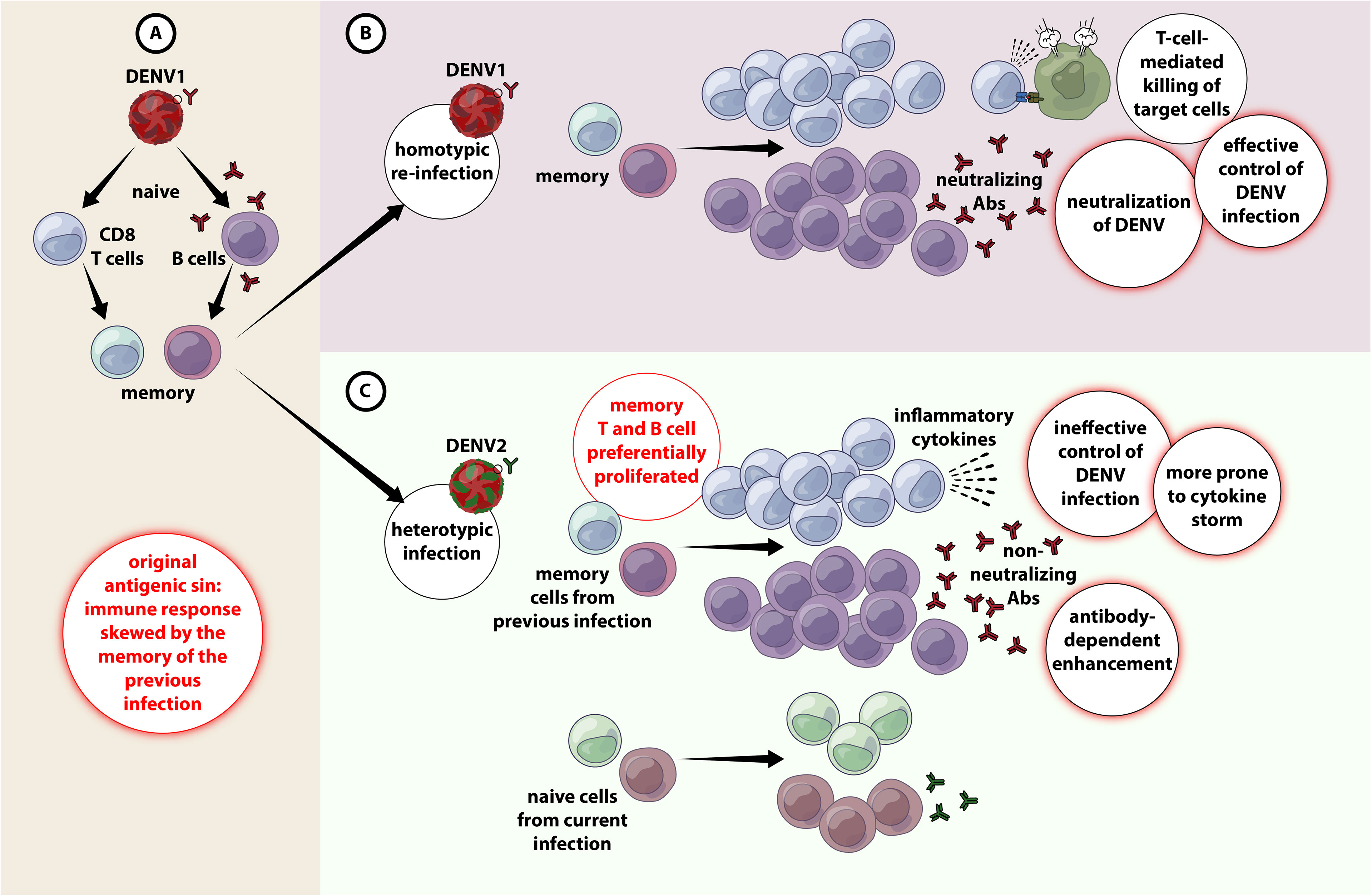 Figure 1 Original antigenic sin and antibody-dependent enhancement in DENV infection. (A) When primary infection occurs with e,g. DENV1, resulting in activation of adaptive immune responses (both T and B cells) DENV1-specific T cells are selected, activated, and clonally expanded to combat infection. Upon termination of primary infection, memory DENV1–specific T cells and B cells are formed and are retained with higher frequency compared to other naïve cells. (B) A secondary infection with the same serotype of DENV (e.g. DENV1) for the second time (homotypic infection), the virus will evoke a memory response that entails in the effective containment of DENV1 by highly specific T and B cell responses. (C) A secondary challenge with a different serotype of DENV (e.g. DENV2) (heterotypic infection), there is a chance that the cross-reactive memory T and B cells get preferentially activated, proliferated over the DENV2-specific T and B cells. The cross-reactive DENV1–specific adaptive immune responses outcompete naïve T cells that would be more specific for DENV2, resulting in an expanded memory T cell pool that is of low specificity for DENV2 and poor viral clearance. Antibody-dependent enhanced replication also has the potential to occur during a secondary, heterologous infection. Akin to T cells, the titer of DENV-specific antibodies produced from prior infection increases substantially during secondary dengue, and they are predominantly non-neutralizing. Binding of these cross-reactive non-neutralizing antibodies with DENV virions could set in motion both extrinsic and intrinsic forms of ADE. Extrinsic ADE occurs when non-neutralizing antibodies forming a virus-antibody complex are recognized and engulfed by other uninfected cells, e.g., monocytes, macrophages, dendritic cells (DCs) and mast cells, via their gamma Fc receptors (FcγR), particularly FcγRI (CD64) and FcγRII (CD32), resulting in an increase in the frequency of DENV-infected cells, and subsequent upsurge in viral production (28, 34). Intrinsic ADE on the other hand, was first observed in Ross River virus (RRV) where incubation of RRV anti-RRV IgG had resulted in ADE-mediated persistent productive infection of macrophages for extended time periods. Further investigations showed that the entry of virus via Fcγ-antibody complexes will bypass TLR3 and TLR7 signaling leading to a Th2-biased immune responses and increased viral production (35, 36). Later the same phenomena was also observed in DENV (37, 38), where viral entry via FcγR often produces 40% of the global population is at risk of infection. Following infection, viruses undergo replication in the local tissues such as the skin, which leads to an activation of a cascade of events including the recruitment of skin resident cells, e.g., Langerhans cells, mast cells, and keratinocytes, and new cells, e.g., T cells and neutrophils, into the site of the infection. After infection of target cells, sensing of viral products results in the activation of innate immune responses, which establish the inflammatory and antiviral state intended to prevent the virus to replicate and spread. However, DENV utilizes several mechanisms to hijack these responses and escape from the normal immune recognition and processing, which results in its dissemination into the lymph nodes. There, DENV further replicates in monocytic cells, resulting in a primary viremia after its systemic disseminated through the circulatory bloodstream. This results in the subsequent infection of peripheral tissues, such as the liver, spleen, and kidney. Evidence also strongly supports the involvement of multiple cell death pathways following DENV infection leading to vascular dysfunction brought about by monocyte activation. Improved understanding of cell death pathways induced by DENV will help in the development of novel modalities of prevention of disease progression. In endemic areas where multiple DENV serotypes circulate, distinct epidemiological studies found that an individual can become exposed to and can have sequential infections with distinct DENV serotypes, which poses a risk of developing severe manifestations such as DHF/DSS. This phenomenon has been attributed to the potential enhancement activity that the pre-existing antibody response elicited from a previous infection with one serotype may have on the infection with a different serotype. This process leads to an increased viral burden that triggers a series of immunological and cellular events, e.g., ADE, hypercytokinemia, skewed T cell responses, and complement pathways, which despite being intended to prevent the viral invasion and infection, can induce host tissue damage leading to pathology and disease. Hence, it is important to study the immunopathology of dengue fever, as we gain more insights into the pathogenic mechanisms of DENV infections, we can hope to improve our efforts towards providing better case management, reduce its overall morbidity and mortality, and assist in the development of safe and effective vaccines against the dreadful disease. Clinician’s CornerMaintenance of adequate hydration is key to dengue management. Patients also must be monitored for warning signs of severe dengue disease, and hence, prompt initiation of early management/treatment intervention is key to preventing dengue-associated complications such as prolonged shock and metabolic acidosis. Hence, the mainstay of successful management includes judicious and timely initiation of IV fluid replacement therapy with isotonic solutions and frequent monitoring of the hemodynamic status and vital signs during the critical phase. Patients should be administered with acetaminophen for pain as well as temperature management. Aspirin and non-steroidal, anti-inflammatory medications could aggravate the bleeding tendency in some patients and, in children, can be associated with the development of Reyes syndrome. Author ContributionsYKY, WFW, RV, IC, and EMS wrote the manuscript. YKY and EMS critically revised the article for important intellectual content, and approved publication of the article. VV, YZ, ML, and HYT provided critical inputs to the manuscript. All authors contributed to the article and approved the submitted version. FundingThis work was supported by Xiamen University Malaysia Research Funding (XMUMRF), (XMUMRF/2018-C2/ILAB/0001) to YKY, (XMUMRF/2020-C5/ITCM/0003) to HYT and (XMURF/2018-C1/ENG/0005) to YZ. VV was supported in part by NIH R01AI148377 (to VV), Emory University CFAR grant P30 AI050409, NIH Office of Research Infrastructure Programs (ORIP) grants P51 OD011132 and U42 OD011023 (to ENPRC). The Swedish Research Council, The Swedish, Physicians against AIDS Research Foundation, The Swedish International Development Cooperation Agency; SIDA SARC, VINNMER for Vinnova, Linköping University Hospital Research Fund, CALF, and The Swedish Society of Medicine (AI52731) to ML, Funding support provided by the Department of Science and Technology-Science and Engineering Research Board, Government of India (CRG/2019/006096) (to EMS). Author DisclaimerThe content is solely the responsibility of the authors and does not necessarily represent the views of the official affiliations of the authors. Conflict of InterestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher’s NoteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. AcknowledgmentsWe appreciate all those who have contributed substantially to the study of dengue immunopathogenesis from the Central University of Tamil Nadu (Jaisheela Vimali), and also from the Department of Microbiology, Government Theni Medical College and Hospitals (Amudhan Murugesan). We thank Rada Ellegård for the scientific editing, writing and support with the illustrations. References1. Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, et al. Global Spread of Dengue Virus Types: Mapping the 70 Year History. Trends Microbiol (2014) 22(3):138–46. doi: 10.1016/j.tim.2013.12.011 PubMed Abstract | CrossRef Full Text | Google Scholar 2. WHO. Dengue and Severe Dengue, Fact Sheets, World Health Organization (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/Dengue-and-severe-Dengue. Google Scholar 3. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The Global Distribution and Burden of Dengue. Nature (2013) 496(7446):504–7. doi: 10.1038/nature12060 PubMed Abstract | CrossRef Full Text | Google Scholar 4. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The Global Burden of Dengue: An Analysis From the Global Burden of Disease Study 2013. Lancet Infect Dis (2016) 16(6):712–23. doi: 10.1016/S1473-3099(16)00026-8 PubMed Abstract | CrossRef Full Text | Google Scholar 5. Fukusumi M, Arashiro T, Arima Y, Matsui T, Shimada T, Kinoshita H, et al. Dengue Sentinel Traveler Surveillance: Monthly and Yearly Notification Trends Among Japanese Travelers, 2006-2014. PLoS Negl Trop Dis (2016) 10(8):e0004924. doi: 10.1371/journal.pntd.0004924 PubMed Abstract | CrossRef Full Text | Google Scholar 6. Simmons CP, Farrar JJ, Nguyen V, Wills B.. Dengue. N Engl J Med (2012) 366(15):1423–32. doi: 10.1056/NEJMra1110265 PubMed Abstract | CrossRef Full Text | Google Scholar 7. What Pay for Performance can Mean for Quality Managers. Healthcare Benchmarks Qual Improv (2004) 11(8):85–8. PubMed Abstract | Google Scholar 8. Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT., et al. Climate Change Influences on Global Distributions of Dengue and Chikungunya Virus Vectors. Philos Trans R Soc Lond B Biol Sci (2015) . 370(1665):20140135. doi: 10.1098/rstb.2014.0135 PubMed Abstract | CrossRef Full Text | Google Scholar 9. Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue Infection. Nat Rev Dis Primers (2016) 2:16055. doi: 10.1038/nrdp.2016.55 PubMed Abstract | CrossRef Full Text | Google Scholar 10. Perera R, Kuhn RJ. Structural Proteomics of Dengue Virus. Curr Opin Microbiol (2008) 11(4):369–77. doi: 10.1016/j.mib.2008.06.004 PubMed Abstract | CrossRef Full Text | Google Scholar 11. Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell (2002) 108(5):717–25. doi: 10.1016/s0092-8674(02)00660-8 PubMed Abstract | CrossRef Full Text | Google Scholar 12. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High Circulating Levels of the Dengue Virus Nonstructural Protein NS1 Early in Dengue Illness Correlate With the Development of Dengue Hemorrhagic Fever. J Infect Dis (2002) 186(8):1165–8. doi: 10.1086/343813 PubMed Abstract | CrossRef Full Text | Google Scholar 13. Kong YY, Thay CH, Tin TC, Devi S.. Rapid Detection, Serotyping and Quantitation of Dengue Viruses by TaqMan Real-Time One-Step RT-PCR. J Virol Methods (2006) 138(1-2):123–30. doi: 10.1016/j.jviromet.2006.08.003 PubMed Abstract | CrossRef Full Text | Google Scholar 14. Yong YK, Thayan R, Chong HT, Tan CT, Sekaran SD.. Rapid Detection and Serotyping of Dengue Virus by Multiplex RT-PCR and Real-Time SYBR Green RT-PCR. Singapore Med J (2007) 48(7):662–8. PubMed Abstract | Google Scholar 15. Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M., et al. Enzyme-Linked Immunosorbent Assay Specific to Dengue Virus Type 1 Nonstructural Protein NS1 Reveals Circulation of the Antigen in the Blood During the Acute Phase of Disease in Patients Experiencing Primary or Secondary Infections. J Clin Microbiol (2002) 40(2):376–81. doi: 10.1128/JCM.40.02.376-381.2002 PubMed Abstract | CrossRef Full Text | Google Scholar 16. Watanabe S, Tan KH, Rathore AP, Rozen-Gagnon K, Shuai W, Ruedl C, et al. The Magnitude of Dengue Virus NS1 Protein Secretion is Strain Dependent and Does Not Correlate With Severe Pathologies in the Mouse Infection Model. J Virol (2012) 86(10):5508–14. doi: 10.1128/JVI.07081-11 PubMed Abstract | CrossRef Full Text | Google Scholar 17. Anand AM, Sistla S, Dhodapkar R, Hamide A, Biswal N, Srinivasan B.. Evaluation of NS1 Antigen Detection for Early Diagnosis of Dengue in a Tertiary Hospital in Southern India. J Clin Diagn Res (2016) 10(4):DC01–4. doi: 10.7860/JCDR/2016/15758.7562 PubMed Abstract | CrossRef Full Text | Google Scholar 18. Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, et al. Vascular Leakage in Severe Dengue Virus Infections: A Potential Role for the Nonstructural Viral Protein NS1 and Complement. J Infect Dis (2006) 193(8):1078–88. doi: 10.1086/500949 PubMed Abstract | CrossRef Full Text | Google Scholar 19. Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, et al. Diagnostic Accuracy of NS1 ELISA and Lateral Flow Rapid Tests for Dengue Sensitivity, Specificity and Relationship to Viraemia and Antibody Responses. PLoS Negl Trop Dis (2009) 3(1):e360. doi: 10.1371/journal.pntd.0000360 PubMed Abstract | CrossRef Full Text | Google Scholar 20. Oliveira ERA, Povoa TF, Nuovo GJ, Allonso D, Salomao NG, Basilio-de-Oliveira CA, et al. Dengue Fatal Cases Present Virus-Specific HMGB1 Response in Peripheral Organs. Sci Rep (2017) 7(1):16011. doi: 10.1038/s41598-017-16197-5 PubMed Abstract | CrossRef Full Text | Google Scholar 21. WHO. WHO Organisation (2009) Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: WHO Organisation (2009). Google Scholar 22. Screaton G, Mongkolsapaya J, Yacoub S, Roberts C.. New Insights Into the Immunopathology and Control of Dengue Virus Infection. Nat Rev Immunol (2015) 15(12):745–59. doi: 10.1038/nri3916 PubMed Abstract | CrossRef Full Text | Google Scholar 23. Halstead SB. Dengvaxia Sensitizes Seronegatives to Vaccine Enhanced Disease Regardless of Age. Vaccine (2017) 35(47):6355–8. doi: 10.1016/j.vaccine.2017.09.089 PubMed Abstract | CrossRef Full Text | Google Scholar 24. Halstead SB. Controversies in Dengue Pathogenesis. Paediatr Int Child Health (2012) 32 Suppl 1:5–9. doi: 10.1179/2046904712Z.00000000045 PubMed Abstract | CrossRef Full Text | Google Scholar 25. Srikiatkhachorn A, Mathew A, Rothman AL. Immune-Mediated Cytokine Storm and its Role in Severe Dengue. Semin Immunopathol (2017) 39(5):563–74. doi: 10.1007/s00281-017-0625-1 PubMed Abstract | CrossRef Full Text | Google Scholar 26. Zompi S, Harris E. Original Antigenic Sin in Dengue Revisited. Proc Natl Acad Sci U S A (2013) 110(22):8761–2. doi: 10.1073/pnas.1306333110 PubMed Abstract | CrossRef Full Text | Google Scholar 27. Rothman AL. Immunity to Dengue Virus: A Tale of Original Antigenic Sin and Tropical Cytokine Storms. Nat Rev Immunol (2011) 11(8):532–43. doi: 10.1038/nri3014 PubMed Abstract | CrossRef Full Text | Google Scholar 28. Narayan R, Tripathi S. Intrinsic ADE: The Dark Side of Antibody Dependent Enhancement During Dengue Infection. Front Cell Infect Microbiol (2020) 10:580096. doi: 10.3389/fcimb.2020.580096 PubMed Abstract | CrossRef Full Text | Google Scholar 29. Guzman MG, Alvarez M, Rodriguez-Roche R, Bernardo L, Montes T, Vazquez S, et al. Neutralizing Antibodies After Infection With Dengue 1 Virus. Emerg Infect Dis (2007) 13(2):282–6. doi: 10.3201/eid1302.060539 PubMed Abstract | CrossRef Full Text | Google Scholar 30. Gallichotte EN, Baric TJ, Nivarthi U, Delacruz MJ, Graham R, Widman DG, et al. Genetic Variation Between Dengue Virus Type 4 Strains Impacts Human Antibody Binding and Neutralization. Cell Rep (2018) 25(5):1214–24. doi: 10.1016/j.celrep.2018.10.006 PubMed Abstract | CrossRef Full Text | Google Scholar 31. Waggoner JJ, Balmaseda A, Gresh L, Sahoo MK, Montoya M, Wang C, et al. Homotypic Dengue Virus Reinfections in Nicaraguan Children. J Infect Dis (2016) 214(7):986–93. doi: 10.1093/infdis/jiw099 PubMed Abstract | CrossRef Full Text | Google Scholar 32. Dong T, Moran E, Vinh Chau N, Simmons C, Luhn K, Peng Y, et al. High Pro-Inflammatory Cytokine Secretion and Loss of High Avidity Cross-Reactive Cytotoxic T-Cells During the Course of Secondary Dengue Virus Infection. PLoS One (2007) 2(12):e1192. doi: 10.1371/journal.pone.0001192 PubMed Abstract | CrossRef Full Text | Google Scholar 33. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, et al. Original Antigenic Sin and Apoptosis in the Pathogenesis of Dengue Hemorrhagic Fever. Nat Med (2003) 9(7):921–7. doi: 10.1038/nm887 PubMed Abstract | CrossRef Full Text | Google Scholar 34. Littaua R, Kurane I, Ennis FA. Human IgG Fc Receptor II Mediates Antibody-Dependent Enhancement of Dengue Virus Infection. J Immunol (1990) 144(8):3183–6. PubMed Abstract | Google Scholar 35. Lidbury BA, Mahalingam S. Specific Ablation of Antiviral Gene Expression in Macrophages by Antibody-Dependent Enhancement of Ross River Virus Infection. J Virol (2000) 74(18):8376–81. doi: 10.1128/jvi.74.18.8376-8381.2000 PubMed Abstract | CrossRef Full Text | Google Scholar 36. Suhrbier A, La Linn M. Suppression of Antiviral Responses by Antibody-Dependent Enhancement of Macrophage Infection. Trends Immunol (2003) 24(4):165–8. doi: 10.1016/s1471-4906(03)00065-6 PubMed Abstract | CrossRef Full Text | Google Scholar 37. Chareonsirisuthigul T, Kalayanarooj S, Ubol S. Dengue Virus (DENV) Antibody-Dependent Enhancement of Infection Upregulates the Production of Anti-Inflammatory Cytokines, But Suppresses Anti-DENV Free Radical and Pro-Inflammatory Cytokine Production, in THP-1 Cells. J Gen Virol (2007) 88(Pt 2):365–75. doi: 10.1099/vir.0.82537-0 PubMed Abstract | CrossRef Full Text | Google Scholar 38. Ubol S, Phuklia W, Kalayanarooj S, Modhiran N.. Mechanisms of Immune Evasion Induced by a Complex of Dengue Virus and Preexisting Enhancing Antibodies. J Infect Dis (2010) 201(6):923–35. doi: 10.1086/651018 PubMed Abstract | CrossRef Full Text | Google Scholar 39. Cruz-Oliveira C, Freire JM, Conceicao TM, Higa LM, Castanho MA, Da Poian AT., et al. Receptors and Routes of Dengue Virus Entry Into the Host Cells. FEMS Microbiol Rev (2015) 39(2):155–70. doi: 10.1093/femsre/fuu004 PubMed Abstract | CrossRef Full Text | Google Scholar 40. Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM.. Intrinsic Antibody-Dependent Enhancement of Microbial Infection in Macrophages: Disease Regulation by Immune Complexes. Lancet Infect Dis (2010) 10(10):712–22. doi: 10.1016/S1473-3099(10)70166-3 PubMed Abstract | CrossRef Full Text | Google Scholar 41. Flipse J, Diosa-Toro MA, Hoornweg TE, van dePol DP, Urcuqui-Inchima S, Smit JM., et al. Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion Against Antiviral Responses. Sci Rep (2016) 6:29201. doi: 10.1038/srep29201 PubMed Abstract | CrossRef Full Text | Google Scholar 42. Anderson R, Wang S, Osiowy C, Issekutz AC.. Activation of Endothelial Cells via Antibody-Enhanced Dengue Virus Infection of Peripheral Blood Monocytes. J Virol (1997) 71(6):4226–32. doi: 10.1128/JVI.71.6.4226-4232.1997 PubMed Abstract | CrossRef Full Text | Google Scholar 43. Kuczera D, Assolini JP, Tomiotto-Pellissier F, Pavanelli WR, Silveira GF.. Highlights for Dengue Immunopathogenesis: Antibody-Dependent Enhancement, Cytokine Storm, and Beyond. J Interferon Cytokine Res (2018) 38(2):69–80. doi: 10.1089/jir.2017.0037 PubMed Abstract | CrossRef Full Text | Google Scholar 44. Uno N, Ross TM. Dengue Virus and the Host Innate Immune Response. Emerg Microbes Infect (2018) 7(1):167. doi: 10.1038/s41426-018-0168-0 PubMed Abstract | CrossRef Full Text | Google Scholar 45. Wu YW, Mettling C, Wu SR, Yu CY, Perng GC, Lin YS, et al. Autophagy-Associated Dengue Vesicles Promote Viral Transmission Avoiding Antibody Neutralization. Sci Rep (2016) 6:32243. doi: 10.1038/srep32243 PubMed Abstract | CrossRef Full Text | Google Scholar 46. Upasani V, Vo HTM, Auerswald H, Laurent D, Heng S, Duong V, et al. Direct Infection of B Cells by Dengue Virus Modulates B Cell Responses in a Cambodian Pediatric Cohort. Front Immunol (2020) 11:594813. doi: 10.3389/fimmu.2020.594813 PubMed Abstract | CrossRef Full Text | Google Scholar 47. Conde JN, Silva EM, Barbosa AS, Mohana-Borges R. The Complement System in Flavivirus Infections. Front Microbiol (2017) 8:213. doi: 10.3389/fmicb.2017.00213 PubMed Abstract | CrossRef Full Text | Google Scholar 48. Thiemmeca S, Tamdet C, Punyadee N, Prommool T, Songjaeng A, Noisakran S, et al. Secreted NS1 Protects Dengue Virus From Mannose-Binding Lectin-Mediated Neutralization. J Immunol (2016) 197(10):4053–65. doi: 10.4049/jimmunol.1600323 PubMed Abstract | CrossRef Full Text | Google Scholar 49. Liu J, Liu Y, Nie K, Du S, Qiu J, Pang X, et al. Flavivirus NS1 Protein in Infected Host Sera Enhances Viral Acquisition by Mosquitoes. Nat Microbiol (2016) 1(9):16087. doi: 10.1038/nmicrobiol.2016.87 PubMed Abstract | CrossRef Full Text | Google Scholar 50. Correa AR, Berbel AC, Papa MP, Morais AT, Pecanha LM, Arruda LB., et al. Dengue Virus Directly Stimulates Polyclonal B Cell Activation. PLoS One (2015) 10(12):e0143391. doi: 10.1371/journal.pone.0143391 PubMed Abstract | CrossRef Full Text | Google Scholar 51. Upasani V, Vo HTM, Ung S, Heng S, Laurent D, Choeung R, et al. Impaired Antibody-Independent Immune Response of B Cells in Patients With Acute Dengue Infection. Front Immunol (2019) 10:2500. doi: 10.3389/fimmu.2019.02500 PubMed Abstract | CrossRef Full Text | Google Scholar 52. Aye KS, Charngkaew K, Win N, Wai KZ, Moe K, Punyadee N, et al. Pathologic Highlights of Dengue Hemorrhagic Fever in 13 Autopsy Cases From Myanmar. Hum Pathol (2014) 45(6):1221–33. doi: 10.1016/j.humpath.2014.01.022 PubMed Abstract | CrossRef Full Text | Google Scholar 53. Yam-Puc JC, Garcia-Cordero J, Calderon-Amador J, Donis-Maturano L, Cedillo-Barron L, Flores-Romo L., et al. Germinal Center Reaction Following Cutaneous Dengue Virus Infection in Immune-Competent Mice. Front Immunol (2015) 6:188. doi: 10.3389/fimmu.2015.00188 PubMed Abstract | CrossRef Full Text | Google Scholar 54. Aguilar-Briseno JA, Upasani V, Ellen BMT, Moser J, Pauzuolis M, Ruiz-Silva M, et al. TLR2 on Blood Monocytes Senses Dengue Virus Infection and its Expression Correlates With Disease Pathogenesis. Nat Commun (2020) 11(1):3177. doi: 10.1038/s41467-020-16849-7 PubMed Abstract | CrossRef Full Text | Google Scholar 55. Kwissa M, Nakaya HI, Onlamoon N, Wrammert J, Villinger F, Perng GC, et al. Dengue Virus Infection Induces Expansion of a CD14(+)CD16(+) Monocyte Population That Stimulates Plasmablast Differentiation. Cell Host Microbe (2014) 16(1):115–27. doi: 10.1016/j.chom.2014.06.001 PubMed Abstract | CrossRef Full Text | Google Scholar 56. Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, et al. Rapid and Massive Virus-Specific Plasmablast Responses During Acute Dengue Virus Infection in Humans. J Virol (2012) 86(6):2911–8. doi: 10.1128/JVI.06075-11 PubMed Abstract | CrossRef Full Text | Google Scholar 57. Balakrishnan T, Bela-Ong DB, Toh YX, Flamand M, Devi S, Koh MB, et al. Dengue Virus Activates Polyreactive, Natural IgG B Cells After Primary and Secondary Infection. PLoS One (2011) 6(12):e29430. doi: 10.1371/journal.pone.0029430 PubMed Abstract | CrossRef Full Text | Google Scholar 58. Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E.. Dominant Cross-Reactive B Cell Response During Secondary Acute Dengue Virus Infection in Humans. PLoS Negl Trop Dis (2012) 6(3):e1568. doi: 10.1371/journal.pntd.0001568 PubMed Abstract | CrossRef Full Text | Google Scholar 59. Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares deMelo KM, McBurney SP, et al. Association Between Magnitude of the Virus-Specific Plasmablast Response and Disease Severity in Dengue Patients. J Immunol (2013) 190(1):80–7. doi: 10.4049/jimmunol.1103350 PubMed Abstract | CrossRef Full Text | Google Scholar 60. Simmons CP, Popper S, Dolocek C, Chau TN, Griffiths M, Dung NT, et al. Patterns of Host Genome-Wide Gene Transcript Abundance in the Peripheral Blood of Patients With Acute Dengue Hemorrhagic Fever. J Infect Dis (2007) 195(8):1097–107. doi: 10.1086/512162 PubMed Abstract | CrossRef Full Text | Google Scholar 61. Rouers A, Chng MHY, Lee B, Rajapakse MP, Kaur K, Toh YX, et al. Immune Cell Phenotypes Associated With Disease Severity and Long-Term Neutralizing Antibody Titers After Natural Dengue Virus Infection. Cell Rep Med (2021) 2(5):100278. doi: 10.1016/j.xcrm.2021.100278 PubMed Abstract | CrossRef Full Text | Google Scholar 62. Godoy-Lozano EE, Tellez-Sosa J, Sanchez-Gonzalez G, Samano-Sanchez H, Aguilar-Salgado A, Salinas-Rodriguez A, et al. Lower IgG Somatic Hypermutation Rates During Acute Dengue Virus Infection is Compatible With a Germinal Center-Independent B Cell Response. Genome Med (2016) 8(1):23. doi: 10.1186/s13073-016-0276-1 PubMed Abstract | CrossRef Full Text | Google Scholar 63. Rouers A, Appanna R, Chevrier M, Lum J, Lau MC. CD27(hi)CD38(hi) Plasmablasts are Activated B Cells of Mixed Origin With Distinct Function. iScience (2021) 24(5):102482. doi: 10.1016/j.isci.2021.102482 PubMed Abstract | CrossRef Full Text | Google Scholar 64. Priyamvada L, Cho A, Onlamoon N, Zheng NY, Huang M, Kovalenkov Y, et al. B Cell Responses During Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts. J Virol (2016) 90(12):5574–85. doi: 10.1128/JVI.03203-15 PubMed Abstract | CrossRef Full Text | Google Scholar 65. Marcial-Juarez E, Garcia-Cordero J, Maqueda-Alfaro RA, Saucedo-Lopez RE, Sanchez-Torres LE, Cedillo- 17 Barron L, et al. Cutaneous Dengue Virus Inoculation Triggers Strong B Cell Reactions But Contrastingly Poor T Cell Responses. Virol Sin (2020) 35(5):575–87. doi: 10.1007/s12250-020-00213-6 PubMed Abstract | CrossRef Full Text | Google Scholar 66. Haltaufderhyde K, Srikiatkhachorn A, Green S, Macareo L, Park S, Kalayanarooj S, et al. Activation of Peripheral T Follicular Helper Cells During Acute Dengue Virus Infection. J Infect Dis (2018) 218(10):1675–85. doi: 10.1093/infdis/jiy360 PubMed Abstract | CrossRef Full Text | Google Scholar 67. Preeyaa SU, et al. Peripheral Follicular T Helper Cells and Mucosal-Associated Invariant T Cells Represent Activated Phenotypes During the Febrile Phase of Acute Dengue Virus Infection. Viral Immunol (2020) 33(9):610–5. doi: 10.1089/vim.2020.0149 PubMed Abstract | CrossRef Full Text | Google Scholar 68. Wijesinghe A, Gamage J, Goonewardena H, Gomes L, Jayathilaka D, Wijeratne DT, et al. Phenotype and Functionality of Follicular Helper T Cells in Patients With Acute Dengue Infection. J BioMed Sci (2020) 27(1):50. doi: 10.1186/s12929-020-00641-2 PubMed Abstract | CrossRef Full Text | Google Scholar 69. Nivarthi UK, Tu HA, Delacruz MJ, Swanstrom J, Patel B, Durbin AP, et al. Longitudinal Analysis of Acute and Convalescent B Cell Responses in a Human Primary Dengue Serotype 2 Infection Model. EBioMedicine (2019) 41:465–78. doi: 10.1016/j.ebiom.2019.02.060 PubMed Abstract | CrossRef Full Text | Google Scholar 70. Woda M, Friberg H, Currier JR, Srikiatkhachorn A, Macareo LR, Green S, et al. Dynamics of Dengue Virus (DENV)-Specific B Cells in the Response to DENV Serotype 1 Infections, Using Flow Cytometry With Labeled Virions. J Infect Dis (2016) 214(7):1001–9. doi: 10.1093/infdis/jiw308 PubMed Abstract | CrossRef Full Text | Google Scholar 71. Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE Jr. Persistence of Circulating Memory B Cell Clones With Potential for Dengue Virus Disease Enhancement for Decades Following Infection. J Virol (2012) 86(5):2665–75. doi: 10.1128/JVI.06335-11 PubMed Abstract | CrossRef Full Text | Google Scholar 72. Appanna R, Kg S, Xu MH, Toh YX, Velumani S, Carbajo D, et al. Plasmablasts During Acute Dengue Infection Represent a Small Subset of a Broader Virus-Specific Memory B Cell Pool. EBioMedicine (2016) 12:178–88. doi: 10.1016/j.ebiom.2016.09.003 PubMed Abstract | CrossRef Full Text | Google Scholar 73. Green S, Rothman A. Immunopathological Mechanisms in Dengue and Dengue Hemorrhagic Fever. Curr Opin Infect Dis (2006) 19(5):429–36. doi: 10.1097/01.qco.0000244047.31135.fa PubMed Abstract | CrossRef Full Text | Google Scholar 74. Nascimento EJ, Hottz ED, Garcia-Bates TM, Bozza F, Marques ET Jr, Barratt-Boyes SM., et al. Emerging Concepts in Dengue Pathogenesis: Interplay Between Plasmablasts, Platelets, and Complement in Triggering Vasculopathy. Crit Rev Immunol (2014) 34(3):227–40. doi: 10.1615/critrevimmunol.2014010212 PubMed Abstract | CrossRef Full Text | Google Scholar 75. Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, et al. Dengue Virus NS1 Protein Activates Cells via Toll-Like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci Transl Med (2015) 7(304):304ra142. doi: 10.1126/scitranslmed.aaa3863 PubMed Abstract | CrossRef Full Text | Google Scholar 76. Muller DA, Young PR. The Flavivirus NS1 Protein: Molecular and Structural Biology, Immunology, Role in Pathogenesis and Application as a Diagnostic Biomarker. Antiviral Res (2013) 98(2):192–208. doi: 10.1016/j.antiviral.2013.03.008 PubMed Abstract | CrossRef Full Text | Google Scholar 77. Young PR, Hilditch PA, Bletchly C, Halloran W.. An Antigen Capture Enzyme-Linked Immunosorbent Assay Reveals High Levels of the Dengue Virus Protein NS1 in the Sera of Infected Patients. J Clin Microbiol (2000) 38(3):1053–7. doi: 10.1128/JCM.38.3.1053-1057.2000 PubMed Abstract | CrossRef Full Text | Google Scholar 78. Yacoub S, Wills B. Predicting Outcome From Dengue. BMC Med (2014) 12:147. doi: 10.1186/s12916-014-0147-9 PubMed Abstract | CrossRef Full Text | Google Scholar 79. Robbiani DF, Ruzek D. A Dark Side to NS1 Antibodies? J Exp Med (2021) 218(9):e20211348. doi: 10.1084/jem.20211348 PubMed Abstract | CrossRef Full Text | Google Scholar 80. Jayathilaka D, Gomes L, Jeewandara C, Jayarathna GSB, Herath D, Perera PA, et al. Role of NS1 Antibodies in the Pathogenesis of Acute Secondary Dengue Infection. Nat Commun (2018) 9(1):5242. doi: 10.1038/s41467-018-07667-z PubMed Abstract | CrossRef Full Text | Google Scholar 81. Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, et al. Generation of IgM Anti-Platelet Autoantibody in Dengue Patients. J Med Virol (2001) 63(2):143–9. doi: 10.1002/1096-9071(20000201)63:23.0.CO;2-L PubMed Abstract | CrossRef Full Text | Google Scholar 82. Adane T, Getawa S. Coagulation Abnormalities in Dengue Fever Infection: A Systematic Review and Meta-Analysis. PLoS Negl Trop Dis (2021) 15(8):e0009666. doi: 10.1371/journal.pntd.0009666 PubMed Abstract | CrossRef Full Text | Google Scholar 83. Patra G, Saha B, Mukhopadhyay S. High Titres of IgM-Bound Circulating Immune Complexes and Erythrocytic Oxidative Damage are Indicators of Dengue Severity. Clin Exp Immunol (2019) 198(2):251–60. doi: 10.1111/cei.13346 PubMed Abstract | CrossRef Full Text | Google Scholar 84. Yong YK, Tan HY, Jen SH, Shankar EM, Natkunam SK, Sathar J, et al. Aberrant Monocyte Responses Predict and Characterize Dengue Virus Infection in Individuals With Severe Disease. J Transl Med (2017) 15(1):121. doi: 10.1186/s12967-017-1226-4 PubMed Abstract | CrossRef Full Text | Google Scholar 85. Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, et al. CLEC5A is Critical for Dengue Virus-Induced Inflammasome Activation in Human Macrophages. Blood (2013) 121(1):95–106. doi: 10.1182/blood-2012-05-430090 PubMed Abstract | CrossRef Full Text | Google Scholar 86. Shrivastava G, Visoso-Carvajal G, Garcia-Cordero J, Leon-Juarez M, Chavez-Munguia B, Lopez T, et al. Dengue Virus Serotype 2 and Its Non-Structural Proteins 2A and 2B Activate NLRP3 Inflammasome. Front Immunol (2020) 11:352. doi: 10.3389/fimmu.2020.00352 PubMed Abstract | CrossRef Full Text | Google Scholar 87. Pan P, Zhang Q, Liu W, Wang W, Yu Z, Lao Z, et al. Dengue Virus Infection Activates Interleukin-1beta to Induce Tissue Injury and Vascular Leakage. Front Microbiol (2019) 10:2637. doi: 10.3389/fmicb.2019.02637 PubMed Abstract | CrossRef Full Text | Google Scholar 88. Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in Health and Disease. Int J Mol Sci (2019) . 20(3):649. doi: 10.3390/ijms20030649 CrossRef Full Text | Google Scholar 89. Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, et al. NLRP3 Inflammasome in Endothelial Dysfunction. Cell Death Dis (2020) 11(9):776. doi: 10.1038/s41419-020-02985-x PubMed Abstract | CrossRef Full Text | Google Scholar 90. Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinrerk W, et al. Secreted NS1 of Dengue Virus Attaches to the Surface of Cells via Interactions With Heparan Sulfate and Chondroitin Sulfate E. PLoS Pathog (2007) 3(11):e183. doi: 10.1371/journal.ppat.0030183 PubMed Abstract | CrossRef Full Text | Google Scholar 91. Dias-Melicio LA, Fernandes RK, Rodrigues DR, Golim MA, Soares AM.. Interleukin-18 Increases TLR4 and Mannose Receptor Expression and Modulates Cytokine Production in Human Monocytes. Mediators Inflamm (2015) 2015:236839. doi: 10.1155/2015/236839 PubMed Abstract | CrossRef Full Text | Google Scholar 92. Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H., et al. The Receptor for Advanced Glycation End Products is Induced by the Glycation Products Themselves and Tumor Necrosis Factor-Alpha Through Nuclear Factor-Kappa B, and by 17beta-Estradiol Through Sp-1 in Human Vascular Endothelial Cells. J Biol Chem (2000) 275(33):25781–90. doi: 10.1074/jbc.M001235200 PubMed Abstract | CrossRef Full Text | Google Scholar 93. Navarro JC, Arrivillaga-Henriquez J, Salazar-Loor J, Rodriguez-Morales AJ.. COVID-19 and Dengue, Co-Epidemics in Ecuador and Other Countries in Latin America: Pushing Strained Health Care Systems Over the Edge. Travel Med Infect Dis (2020) 37:101656. doi: 10.1016/j.tmaid.2020.101656 PubMed Abstract | CrossRef Full Text | Google Scholar 94. Lam LTM, Chua YX, Tan DHY. Roles and Challenges of Primary Care Physicians Facing a Dual Outbreak of COVID-19 and Dengue in Singapore. Fam Pract (2020) 37(4):578–9. doi: 10.1093/fampra/cmaa047 PubMed Abstract | CrossRef Full Text | Google Scholar 95. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell (2020) 181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026 PubMed Abstract | CrossRef Full Text | Google Scholar 96. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest (2020) 130(5):2620–9. doi: 10.1172/JCI137244 PubMed Abstract | CrossRef Full Text | Google Scholar 97. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R.. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol (2020) 11:1446. doi: 10.3389/fimmu.2020.01446 PubMed Abstract | CrossRef Full Text | Google Scholar 98. Lin SH, Zhao YS, Zhou DX, Zhou FC, Xu F.. Coronavirus Disease 2019 (COVID-19): Cytokine Storms, Hyper-Inflammatory Phenotypes, and Acute Respiratory Distress Syndrome. Genes Dis (2020) 7(4):520–7. doi: 10.1016/j.gendis.2020.06.009 PubMed Abstract | CrossRef Full Text | Google Scholar 99. Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS., et al. Cellular and Cytokine Correlates of Severe Dengue Infection. PLoS One (2012) 7(11):e50387. doi: 10.1371/journal.pone.0050387 PubMed Abstract | CrossRef Full Text | Google Scholar 100. Nguyen TH, Nguyen TH, Vu TT, Farrar J, Hoang TL, Dong TH, et al. Corticosteroids for Dengue - Why Don't They Work? PLoS Negl Trop Dis (2013) 7(12):e2592. doi: 10.1371/journal.pntd.0002592 PubMed Abstract | CrossRef Full Text | Google Scholar 101. Tramontini Gomes de Sousa Cardozo F, Baimukanova G, Lanteri MC, Keating SM, Moraes Ferreira F, Heitman 56 J, et al. Serum From Dengue Virus-Infected Patients With and Without Plasma Leakage Differentially Affects Endothelial Cells Barrier Function In Vitro. PLoS One (2017) 12(6):e0178820. doi: 10.1371/journal.pone.0178820 PubMed Abstract | CrossRef Full Text | Google Scholar 102. Dayarathna S, Jeewandara C, Gomes L, Somathilaka G, Jayathilaka D, Vimalachandran V, et al. Similarities and Differences Between the 'Cytokine Storms' in Acute Dengue and COVID-19. Sci Rep (2020) 10(1):19839. doi: 10.1038/s41598-020-76836-2 PubMed Abstract | CrossRef Full Text | Google Scholar 103. Kamaladasa A, Gomes L, Jeewandara C, Shyamali NL, Ogg GS, Malavige GN., et al. Lipopolysaccharide Acts Synergistically With the Dengue Virus to Induce Monocyte Production of Platelet Activating Factor and Other Inflammatory Mediators. Antiviral Res (2016) 133:183–90. doi: 10.1016/j.antiviral.2016.07.016 PubMed Abstract | CrossRef Full Text | Google Scholar 104. Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, et al. Plasma IP-10 and MCP-3 Levels are Highly Associated With Disease Severity and Predict the Progression of COVID-19. J Allergy Clin Immunol (2020) 146(1):119–27.e4. doi: 10.1016/j.jaci.2020.04.027 PubMed Abstract | CrossRef Full Text | Google Scholar 105. Wu D, Lu J, Liu Q, Ma X, He W.. To Alert Coinfection of COVID-19 and Dengue Virus in Developing Countries in the Dengue-Endemic Area. Infect Control Hosp Epidemiol (2020) 41(12):1482. doi: 10.1017/ice.2020.187 PubMed Abstract | CrossRef Full Text | Google Scholar 106. Gong J, Dong H, Xia QS, Huang ZY, Wang DK, Zhao Y, et al. Correlation Analysis Between Disease Severity and Inflammation-Related Parameters in Patients With COVID-19: A Retrospective Study. BMC Infect Dis (2020) 20(1):963. doi: 10.1186/s12879-020-05681-5 PubMed Abstract | CrossRef Full Text | Google Scholar 107. Malibari AA, Al-Husayni F, Jabri A, Al-Amri A, Alharbi M.. A Patient With Dengue Fever and COVID-19: Coinfection or Not? Cureus (2020) 12(12):e11955. doi: 10.7759/cureus.11955 PubMed Abstract | CrossRef Full Text | Google Scholar 108. Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Wangdi K.. Clinical Features and Outcomes of COVID-19 and Dengue Co-Infection: A Systematic Review. BMC Infect Dis (2021) 21(1):729. doi: 10.1186/s12879-021-06409-9 PubMed Abstract | CrossRef Full Text | Google Scholar 109. Zheng W, Wu H, Liu C, Yan Q, Wang T, Wu P, et al. Identification of COVID-19 and Dengue Host Factor Interaction Networks Based on Integrative Bioinformatics Analyses. Front Immunol (2021) 12:707287. doi: 10.3389/fimmu.2021.707287 PubMed Abstract | CrossRef Full Text | Google Scholar 110. Teotonio I, de Carvalho JL, Castro LC, Nitz N, Hagstrom L, Rios GG, et al. Clinical and Biochemical Parameters of COVID-19 Patients With Prior or Active Dengue Fever. Acta Trop (2021) 214:105782. doi: 10.1016/j.actatropica.2020.105782 PubMed Abstract | CrossRef Full Text | Google Scholar 111. Yan G, Lee CK, Lam LTM, Yan B, Chua YX, Lim AYN, et al. Covert COVID-19 and False-Positive Dengue Serology in Singapore. Lancet Infect Dis (2020) 20(5):536. doi: 10.1016/S1473-3099(20)30158-4 PubMed Abstract | CrossRef Full Text | Google Scholar 112. Joob B, Wiwanitkit V. COVID-19 can Present With a Rash and be Mistaken for Dengue. J Am Acad Dermatol (2020) 82(5):e177. doi: 10.1016/j.jaad.2020.03.036 PubMed Abstract | CrossRef Full Text | Google Scholar 113. Santoso MS, Masyeni S, Haryanto S, Yohan B, Hibberd ML, Sasmono RT., et al. Assessment of Dengue and COVID-19 Antibody Rapid Diagnostic Tests Cross-Reactivity in Indonesia. Virol J (2021) 18(1):54. doi: 10.1186/s12985-021-01522-2 PubMed Abstract | CrossRef Full Text | Google Scholar 114. Nath H, Mallick A, Roy S, Sukla S, Biswas S.. Computational Modelling Supports That Dengue Virus Envelope Antibodies can Bind to SARS-CoV-2 Receptor Binding Sites: Is Pre-Exposure to Dengue Virus Protective Against COVID-19 Severity? Comput Struct Biotechnol J (2021) 19:459–66. doi: 10.1016/j.csbj.2020.12.037 PubMed Abstract | CrossRef Full Text | Google Scholar 115. Silvestre OM, Costa LR, Lopes BVR, Barbosa MR, Botelho KKP, Albuquerque KLC, et al. Previous Dengue Infection and Mortality in COVID-19. Clin Infect Dis (2020). doi: 10.1093/cid/ciaa1895 CrossRef Full Text | Google Scholar 116. Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective Efficacy of the Recombinant, Live-Attenuated, CYD Tetravalent Dengue Vaccine in Thai Schoolchildren: A Randomised, Controlled Phase 2b Trial. Lancet (2012) 380(9853):1559–67. doi: 10.1016/S0140-6736(12)61428-7 PubMed Abstract | CrossRef Full Text | Google Scholar 117. Mahalingam S, Herring BL, Halstead SB. Call to Action for Dengue Vaccine Failure. Emerg Infect Dis (2013) 19(8):1335–7. doi: 10.3201/eid1908.121864 PubMed Abstract | CrossRef Full Text | Google Scholar 118. Wilder-Smith A, Flasche S, Smith PG. Vaccine-Attributable Severe Dengue in the Philippines. Lancet (2019) 394(10215):2151–2. doi: 10.1016/S0140-6736(19)32525-5 PubMed Abstract | CrossRef Full Text | Google Scholar 119. FDA Expands Authorization of Two Monoclonal Antibodies for Treatment and Post-Exposure Prevention of COVID-19 to Younger Pediatric Patients, Including Newborns (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-expands-authorization-two-monoclonal-antibodies-treatment-and-post-exposure-prevention-covid-19. Google Scholar 120. Olkowski S, Forshey BM, Morrison AC, Rocha C, Vilcarromero S, Halsey ES, et al. Reduced Risk of Disease During Postsecondary Dengue Virus Infections. J Infect Dis (2013) 208(6):1026–33. doi: 10.1093/infdis/jit273 PubMed Abstract | CrossRef Full Text | Google Scholar 121. Wan SW, Chen PW, Chen CY, Lai YC, Chu YT, Hung CY, et al. Therapeutic Effects of Monoclonal Antibody Against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection. J Immunol (2017) 199(8):2834–44. doi: 10.4049/jimmunol.1601523 PubMed Abstract | CrossRef Full Text | Google Scholar 122. O'Donnell KL, Espinosa DA, Puerta-Guardo H, Biering SB, Warnes CM, Schiltz J, et al. Avian Anti-NS1 IgY Antibodies Neutralize Dengue Virus Infection and Protect Against Lethal Dengue Virus Challenge. Antiviral Res (2020) 183:104923. doi: 10.1016/j.antiviral.2020.104923 PubMed Abstract | CrossRef Full Text | Google Scholar 123. Kang TH, Jung ST. Boosting Therapeutic Potency of Antibodies by Taming Fc Domain Functions. Exp Mol Med (2019) 51(11):1–9. doi: 10.1038/s12276-019-0345-9 PubMed Abstract | CrossRef Full Text | Google Scholar 124. Bournazos S, Gupta A, Ravetch JV. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat Rev Immunol (2020) 20(10):633–43. doi: 10.1038/s41577-020-00410-0 PubMed Abstract | CrossRef Full Text | Google Scholar 125. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic-Uremic Syndrome. N Engl J Med (2013) 368(23):2169–81. doi: 10.1056/NEJMoa1208981 PubMed Abstract | CrossRef Full Text | Google Scholar 126. Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, et al. Eculizumab Reduces Complement Activation, Inflammation, Endothelial Damage, Thrombosis, and Renal Injury Markers in aHUS. Blood (2015) 125(21):3253–62. doi: 10.1182/blood-2014-09-600411 PubMed Abstract | CrossRef Full Text | Google Scholar 127. Carr JM, Cabezas-Falcon S, Dubowsky JG, Hulme-Jones J, Gordon DL.. Dengue Virus and the Complement Alternative Pathway. FEBS Lett (2020) 594(16):2543–55. doi: 10.1002/1873-3468.13730 PubMed Abstract | CrossRef Full Text | Google Scholar 128. Tapia-Abellan A, Angosto-Bazarra D, Martinez-Banaclocha H, de Torre-Minguela C, Ceron-Carrasco JP, Perez-Sanchez H, et al. MCC950 Closes the Active Conformation of NLRP3 to an Inactive State. Nat Chem Biol (2019) 15(6):560–4. doi: 10.1038/s41589-019-0278-6 PubMed Abstract | CrossRef Full Text | Google Scholar 129. Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, et al. MCC950 Directly Targets the NLRP3 ATP-Hydrolysis Motif for Inflammasome Inhibition. Nat Chem Biol (2019) 15(6):556–9. doi: 10.1038/s41589-019-0277-7 PubMed Abstract | CrossRef Full Text | Google Scholar 130. Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A Small-Molecule Inhibitor of the NLRP3 Inflammasome for the Treatment of Inflammatory Diseases. Nat Med (2015) 21(3):248–55. doi: 10.1038/nm.3806 PubMed Abstract | CrossRef Full Text | Google Scholar 131. Dinarello CA, Novick D, Kim S, Kaplanski G.. Interleukin-18 and IL-18 Binding Protein. Front Immunol (2013) 4:289 DOI|. doi: 10.3389/fimmu.2013.00289 CrossRef Full Text | Google Scholar 132. Li X, Cui W, Hull L, Wang L, Yu T, Xiao M., et al. IL-18 Binding Protein (IL-18BP) as a Novel Radiation Countermeasure After Radiation Exposure in Mice. Sci Rep (2020) 10(1):18674. doi: 10.1038/s41598-020-75675-5 PubMed Abstract | CrossRef Full Text | Google Scholar 133. Potts JA, Rothman AL. Clinical and Laboratory Features That Distinguish Dengue From Other Febrile Illnesses in Endemic Populations. Trop Med Int Health (2008) 13(11):1328–40. doi: 10.1111/j.1365-3156.2008.02151.x PubMed Abstract | CrossRef Full Text | Google Scholar 134. Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA.. Platelets: Versatile Effector Cells in Hemostasis, Inflammation, and the Immune Continuum. Semin Immunopathol (2012) 34(1):5–30. doi: 10.1007/s00281-011-0286-4 PubMed Abstract | CrossRef Full Text | Google Scholar 135. Nachman RL, Rafii S. Platelets, Petechiae, and Preservation of the Vascular Wall. N Engl J Med (2008) 359(12):1261–70. doi: 10.1056/NEJMra0800887 PubMed Abstract | CrossRef Full Text | Google Scholar 136. Stokes KY, Granger DN. Platelets: A Critical Link Between Inflammation and Microvascular Dysfunction. J Physiol (2012) 590(5):1023–34. doi: 10.1113/jphysiol.2011.225417 PubMed Abstract | CrossRef Full Text | Google Scholar 137. Ojha A, Nandi D, Batra H, Singhal R, Annarapu GK, Bhattacharyya S, et al. Platelet Activation Determines the Severity of Thrombocytopenia in Dengue Infection. Sci Rep (2017) 7:41697. doi: 10.1038/srep41697 PubMed Abstract | CrossRef Full Text | Google Scholar 138. Quach ME, Chen W, Li R. Mechanisms of Platelet Clearance and Translation to Improve Platelet Storage. Blood (2018) 131(14):1512–21. doi: 10.1182/blood-2017-08-743229 PubMed Abstract | CrossRef Full Text | Google Scholar 139. Djamiatun K, van derVen AJ, de Groot PG, Faradz SM, Hapsari D, Dolmans WM, et al. Severe Dengue is Associated With Consumption of Von Willebrand Factor and Its Cleaving Enzyme ADAMTS-13. PLoS Negl Trop Dis (2012) 6(5):e1628. doi: 10.1371/journal.pntd.0001628 PubMed Abstract | CrossRef Full Text | Google Scholar 140. Ward S, O'Sullivan JM, O'Donnell JS. Von Willebrand Factor Sialylation-A Critical Regulator of Biological Function. J Thromb Haemost (2019) 17(7):1018–29. doi: 10.1111/jth.14471 PubMed Abstract | CrossRef Full Text | Google Scholar 141. Glanz VY, Myasoedova VA, Grechko AV, Orekhov AN.. Inhibition of Sialidase Activity as a Therapeutic Approach. Drug Des Devel Ther (2018) 12:3431–7. doi: 10.2147/DDDT.S176220 PubMed Abstract | CrossRef Full Text | Google Scholar 142. WHO. Vaccines and Immunization: Dengue (2018). Available at: https://www.who.int/news-room/questions-and-answers/item/dengue-vaccines. Google Scholar 143. Mullard A. NLRP3 Inhibitors Stoke Anti-Inflammatory Ambitions. Nat Rev Drug Discov (2019) 18(6):405–7. doi: 10.1038/d41573-019-00086-9 PubMed Abstract | CrossRef Full Text | Google Scholar GlossaryAlarmins: Also called as danger-associated molecular patterns, such as IL-33, Hsp70, HMGB1 and IL-1α, are released by injured or necrotic cells Antibody-dependent cellular cytotoxicity: ADCC is an immune mechanism where an FcR-bearing effector cell recognizes and binds to Fc fragment of an antibody molecule already bound to an antigen displayed on a target cell (for instance, a host cell expressing pathogen-derived antigens on its surface), which entails in downstream signaling within the effector cell resulting in cytolytic granule release and consequent lysis of the target cell via the perforin-granzyme pathway. Antibody-dependent enhancement: ADE represents a mechanism where a non-neutralizing antibody becomes bound to a viral particle (often during secondary infection) is recognized by the Fc gamma receptor IIa (FcγRIIa) expressed on a phagocytic cell leading to enhanced intracellular viral replication, or immune complex formation resulting in hyperinflammation and immunopathology. ADE occur when binding antibodies (non-neutralizing) or antibodies at sub-optimal levels bind to viral particles without necessitating viral clearance. Atypical hemolytic uremic syndrome: A rare life-threatening disease resulting from dysregulated activation of the complement system culminating in the formation of thrombi in small blood vessels of visceral organs especially the kidneys progressing to end-stage renal disease (ESRD). Cytokine: A wide range of proteins secreted by various host cells especially immune cells that facilitate intercellular signaling, and can exert localized or systemic biological effects. Cytokine storm: A state of excessive systemic inflammation involving dramatically elevated levels of proinflammatory cytokines and inflammatory cells viz. macrophages, neutrophils, mast cells, eosinophils and basophils. Cytokine release syndrome can often result in the dysfunction of secondary organs, systemic multi-organ failure, and can be fatal. Damage-associated molecular patterns (DAMPs): DAMPs are molecules released by injured and necrotized cells usually following an infection in the host, and are recognized by pattern recognition receptors (PRRs) expressed on host cells to activate innate immune responses. Defervescence: A phase in dengue fever where the patient’s body temperature decreases rapidly. Dengue hemorrhagic fever: DHF is currently defined by four WHO criteria viz., (1) Fever or recent history of fever lasting 2–7 days (2) Any hemorrhagic manifestation (3) Thrombocytopenia (platelet count of |
【本文地址】
今日新闻 |
推荐新闻 |